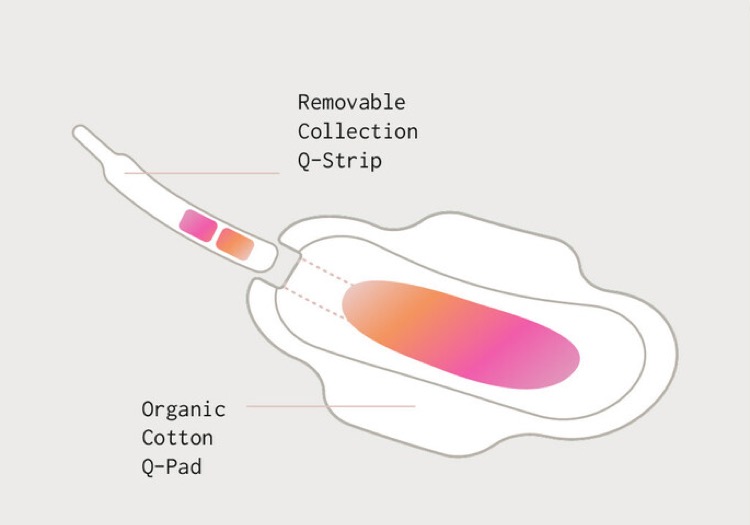
SAN FRANCISCO - Qvin, the biotechnology research company that developed the first healthcare service that collects menstrual blood samples as an alternative to traditionally collected venous blood draws, has received FDA clearance for its Q-Pad and A1c Test.
The clearance makes it possible for the millions of women in America who live with diabetes to receive monitoring of A1c, using laboratory tests performed on the Q-Pad. More broadly, this marks an opportunity for testing important biomarkers for the more than 80 million people who menstruate in the US.